The environmental persistence of per- and polyfluoroalkyl substances (PFAS) has emerged as a critical challenge for modern science. Since the 1950s, this massive group of over 14,000 synthetic chemicals has been integrated into a vast array of domestic and commercial products. Their defining characteristic—the carbon-fluorine bond—is the strongest link in organic chemistry, rendering them effectively non-biodegradable and earning them the moniker “forever chemicals” Due to their high water solubility and mobility, PFAS have successfully infiltrated soil, water, and biological systems globally. Breaking these molecules down requires high operating temperatures to overcome their inherent thermodynamic stability. Consequently, developing sustainable, cost-effective methods for their removal from wastewater and biosolids is a vital ecological priority.
The Mechanics of Biochar as a Remediation Tool
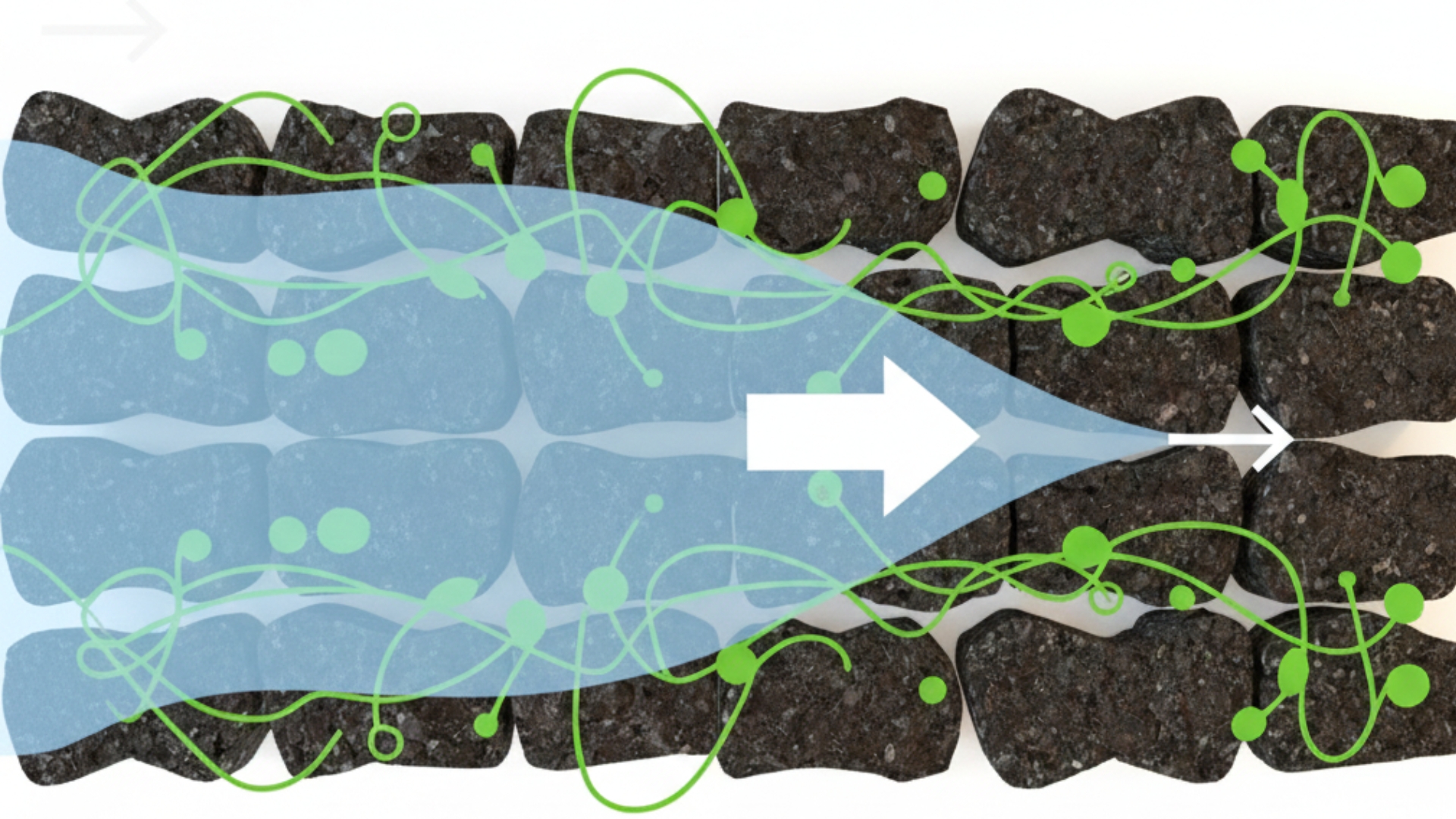
BiocharBiochar is a carbon-rich material created from biomass decomposition in low-oxygen conditions. It has important applications in environmental remediation, soil improvement, agriculture, carbon sequestration, energy storage, and sustainable materials, promoting efficiency and reducing waste in various contexts while addressing climate change challenges. More produced through the thermal decomposition of organic matter in an oxygen-limited environment. It is increasingly recognized as a superior alternative to traditional treatment methods because it aligns with circular economy principles by recycling waste materials into high-value environmental products. The material’s high surface area, developed pore structure, and high cation-exchange capacity make it an exceptional medium for extracting PFAS from contaminated water. The adsorption process is governed by a complex interplay of hydrophobic and electrostatic interactions, supplemented by ion exchange and physical pore filling. Furthermore, modified biochar materials can enhance these effects through surface functionalization and increased carbon content.
Feedstock Diversity and Production Methodology
The versatility of biochar begins with its synthesis from diverse biomassBiomass is a complex biological organic or non-organic solid product derived from living or recently living organism and available naturally. Various types of wastes such as animal manure, waste paper, sludge and many industrial wastes are also treated as biomass because like natural biomass these More feedstocks, ranging from agricultural residues and aquatic weeds to abattoir waste. Research has demonstrated successful biochar production using materials such as rice husk, cow dung, animal bone, water hyacinth, and bamboo.
While controlled pyrolysisPyrolysis is a thermochemical process that converts waste biomass into bio-char, bio-oil, and pyro-gas. It offers significant advantages in waste valorization, turning low-value materials into economically valuable resources. Its versatility allows for tailored products based on operational conditions, presenting itself as a cost-effective and efficient More is the primary production technique, alternative methods like gasificationGasification is a high-temperature, thermochemical process that converts carbon-based materials into a gaseous fuel called syngas and solid by-products. It takes place in an oxygen-deficient environment at temperatures typically above 750°C. Unlike combustion, which fully burns material to produce heat and carbon dioxide (CO2), gasification More and hydrothermal carbonization are effective for treating organic waste and sludge to produce biochar with enhanced oxygen-containing functional groups, which further boosts adsorption capacity. To maximize performance, biochars undergo activation; physical activation involves oxidation with steam or CO2 at high temperatures, while chemical activation utilizes agents like KOH, ZnCl2, or H2PO4 at lower temperatures.
Characterization and Performance Evaluation
To ensure effectiveness in remediation, advanced analytical tools are employed to characterize biochar’s properties. Surface morphology is examined via SEM and TEM, while porosityPorosity of biochar is a key factor in its effectiveness as a soil amendment and its ability to retain water and nutrients. Biochar’s porosity is influenced by feedstock type and pyrolysis temperature, and it plays a crucial role in microbial activity and overall soil health. Biochar More and surface area are quantified through BET analysis. Elemental composition and chemical functional groups are identified using XPS, EDS, FTIR, and Raman spectroscopy, and thermal stability is assessed via TGA. In practical application, adsorption efficiency is determined by batch experiments that evaluate variables such as PFAS concentration, contact time, adsorbent dosage, temperature, and pHpH is a measure of how acidic or alkaline a substance is. A pH of 7 is neutral, while lower pH values indicate acidity and higher values indicate alkalinity. Biochars are normally alkaline and can influence soil pH, often increasing it, which can be beneficial More. Evidence suggests that acidic to neutral pH levels typically favor adsorption, whereas high pH levels can trigger desorption.
Comparative Analysis of Adsorbent Technologies
The selection of an appropriate adsorbent for PFAS remediation involves a critical trade-off between technical efficiency and operational viability. Activated CarbonActivated carbon is a form of carbon that has been processed to create a vast network of tiny pores, increasing its surface area significantly. This extensive surface area makes activated carbon exceptionally effective at trapping and holding impurities, like a molecular sponge. It is commonly More remains the most established industry standard due to its wide availability and historical usage, yet it frequently suffers from low removal efficiency when targeting short-chain PFAS. In contrast, Biochar presents a highly sustainable and cost-effective alternative that aligns with circular economy goals, though it often necessitates specific surface modifications to reach peak performance levels.
For more specialized applications, Ion-Exchange Resins offer high efficiency across the entire spectrum of PFAS molecules, but their adoption is often limited by high procurement costs and the necessity for complex regeneration processes. Newer materials like Metal Organic Frameworks provide exceptionally large surface areas for adsorption but face significant challenges regarding large-scale industrial production. Finally, Hybrid Adsorbents allow for precise customization to target specific PFAS compounds, though this specificity comes at the cost of expensive and complex synthesis procedures.
Analytical Precision and Conclusion
The quantification of PFAS at minute concentrations is achieved through high-precision analytical techniques. Liquid Chromatography-Electrospray Ionization Mass Spectrometry is commonly used for detecting PFAS in water and adsorbents, while Ultra-Performance Liquid Chromatography-High Resolution Mass Spectrometry can provide quantification at the nanogram level. Additionally, fluoride ion-selective electrodes are used to measure defluorination efficiency in degradation studies.
In conclusion, biochar-based materials offer a scientifically robust and eco-friendly solution to the PFAS crisis. By leveraging high surface area and resistance to biodegradation, biochar provides a sustainable pathway for long-term environmental remediation.
References
Aboughaly, M., & Fattah, ; I M R. (2023). Production of Biochar from Biomass Pyrolysis for Removal of
PFAS from Wastewater and Biosolids: A Critical Review. Preprint, 1(1), 1–11.
https://doi.org/10.20944/preprints202304.0309.v1
Behnami, A., Pourakbar, M., Ayyar, A. S. R., Lee, J. W., Gagnon, G., & Zoroufchi Benis, K. (2024).
Treatment of aqueous per- and poly-fluoroalkyl substances: A review of biochar adsorbent preparation methods. Chemosphere, 357(January), 142088. https://doi.org/10.1016/j.chemosphere.2024.142088
Ezeorba, T. P. C., Okeke, E. S., Nwankwo, C. E., Emencheta, S. C., Enochoghene, A. E., Okeke, V. C., &
Ozougwu, V. E. O. (2024). Emerging eco-friendly technologies for remediation of Per- and polyfluoroalkyl substances (PFAS) in water and wastewater: A pathway to environmental sustainability. Chemosphere, 364(August), 143168. https://doi.org/10.1016/j.chemosphere.2024.143168
Hakeem, I. G., Halder, P., Patel, S., Selezneva, E., Rathnayake, N., Marzbali, M. H., Veluswamy, G., Sharma,
A., Kundu, S., Surapaneni, A., Megharaj, M., Batstone, D. J., & Shah, K. (2024). Current understanding
on the transformation and fate of per- and polyfluoroalkyl substances before, during, and after thermal treatment of biosolids. Chemical Engineering Journal, 493(May), 152537.
https://doi.org/10.1016/j.cej.2024.152537
Kumar, A., Shaikh, W. A., Maqsood, H. M., Parikh, S. J., & Biswas, J. K. (2025). Harnessing Sustainable
Biochar-based Composites for Effective PFAS Removal from Wastewater. Current Opinion in
Environmental Science & Health, 43, 100594. https://doi.org/10.1016/j.coesh.2025.100594
Saravanan, A., & Kumar, P. S. (2022). Biochar derived carbonaceous material for various environmental
applications: Systematic review. Environmental Research, 214(P1), 113857.
https://doi.org/10.1016/j.envres.2022.113857
Song, Z., He, J., Kouzehkanan, S. M. T., Oh, T. S., Olshansky, Y., Duin, E. C., Carroll, K. C., & Wang, D.
(2024). Enhanced sorption and destruction of PFAS by biochar-enabled advanced reduction process.
Chemosphere, 363(May), 142760. https://doi.org/10.1016/j.chemosphere.2024.142760





Leave a Reply