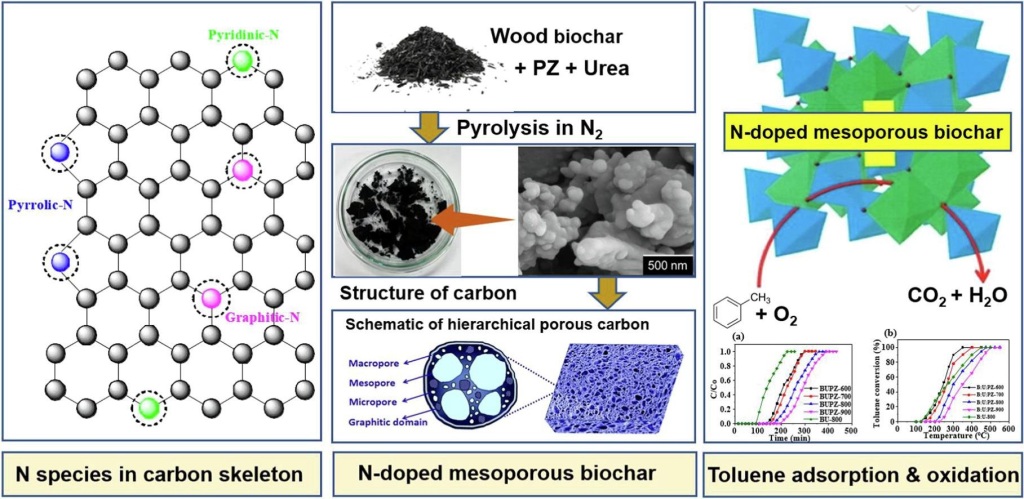
A recent study explored the potential of nitrogen-doped mesoporous biochar for advanced toluene remediation through adsorption and catalytic oxidation. This research, led by Md. Wahad Uz Zaman and colleagues, utilized a salt-templating method to synthesize biochar with varying nitrogen contents and mesoporous structures. Key findings revealed that the synthesis temperature and nitrogen functionalization critically influenced the biochar’s toluene removal performance.
The biochar sample synthesized at 900°C (BUPZ-900) demonstrated an outstanding toluene adsorption capacity of 283.85 mg/g. This impressive performance was attributed to its high surface area and mesopore volume, enhanced by the presence of graphitic nitrogen, which facilitated adsorption through π-π stacking and van der Waals interactions. In contrast, another sample synthesized at 600°C (BUPZ-600), with a high nitrogen content and predominant pyridinic-N, exhibited the highest catalytic activity for toluene oxidation. The basicity of pyridinic-N provided active sites for this process, despite the lower surface area of BUPZ-600.
The study emphasizes that nitrogen-doped mesoporous biochar can serve as an effective adsorbent or catalyst for volatile organic compounds (VOCs) like toluene. These findings are significant as they suggest a metal-free solution for environmental remediation, which could be both efficient and cost-effective. The research highlights the importance of optimizing synthesis conditions and nitrogen functionalization to enhance the performance of biochar in environmental applications.





Leave a comment